Description
Buy Wholesale Healgen Orient Gene Lateral Flow Tests for your Company
Buy & Protect your staff with these Healgen Lateral Flow antigen tests for COVID-19. Available in 20, Single & 5 Packs. This test underwent an extensive validation process with Public Health England, results of which at low and high viral loads can be seen below.
Source: Manufacturer Statement
Why Healgen (Orient Gene)?
This test, a version of which has also been distributed by the UK NHS has a very high specificity – a key measure of the reliability of a positive test result. A very high specificity indicates a low number of false positive results caused by other reactive pathogens, reducing unnecessary isolations and disruptions to your company operations. Healgen was one of the first tests to be validated by the UK Government’s approval process, receiving approval in October 2020.
Known also as the Orient Gene Test, this is in use by Councils, Businesses & the NHS for asymptomatic screening. Developed and manufactured by Healgen Scientific of Houston, Texas & Zhejiang Orient Gene Biotech Co.,China this highly sensitive, specific and accurate antigen test gives a result in just 15 minutes. The swab method is simple with only a Nasal Swab being required that can be performed by the employee without supervision, no swabbing of the throat and tonsils is needed. The test kit contains a procedure sheet which advises the user on how to interpret the results with advice on any further action to take. View a sample of the instructions below.
Free Next Working Day Delivery when ordering 2 or more* – *Order before 2PM for same day dispatch Mon-Fri.
Jump to results of evaluation performance data.
Comes with 20 tubes pre-filled with liquid buffer solution.
In addition to the UK Government & MHRA Approval status, the Healgen antigen test for Covid-19 is listed on the European Union Health Security Committee’s common list of rapid antigen tests for member states.
Note: Item non-returnable due to UK Regulations – returns



UK Government Approval Certification

“Public Health England Porton Down subsequently performed phase 3 testing to assess whether the lateral flow devices that passed phase 2 displayed performance characteristics desirable for mass population, community-based testing. The desirable performance characteristics are:
- very high specificity
- very high sensitivity against viral loads associated with infectiousness
”
– The Healgen Lateral Flow device displayed the desirable performance characteristics and passed phase 3 validation with Public Health England. Source: GOV.UK (Link)
Please note: Privately purchased tests cannot be registered on the NHS app, please contact NHS Test & Trace if this is your requirement. This product offering is not suitable for travel testing. Please read the note below for more information.
Note on Travel Tests:
Please note for travel purposes many countries require certification of a test that is purchased from and performed by or under the supervision of a Medical Clinic. We do not offer this service. (These are exempt from returns.) We do not support testing for travel purposes or day 2.
Note on NHS App/Gov.UK website:
Only tests supplied from NHS test and trace can be used to input results into the NHS App or Gov.UK website, please refer to NHS provided kits only for this purpose (e.g. from your local pharmacy). All privately provided tests are not compatible with the NHS App and website.
Key Facts & Performance Data
- Rapid Testing for the SARS-CoV-2 antigen within the first ten days of symptom onset, and asymptomatic individuals.
- Rapid Results within 15 minutes.
- Facilitates patient treatment decisions quickly
- Simple, time-saving procedure
- No instrumentation required.
- High Sensitivity and Specificity
- Storage Temperature: 2-30°C
- Shelf Life: 24 months from date of manufacture
- Note: Negative results do not rule out SARS-CoV-2 infection, particularly those who have been in contact with the virus. Follow up testing with a molecular diagnostic test (e.g. PCR) should be considered to rule out infection in these individuals.
Performance Characteristics provided by the manufacturer (Healgen / Orient Gene) for symptomatic subjects (i.e. Diagnostic Sensitivity)
Please see the note below the performance results for details of the study and information regarding the sensitivity for asymptomatic individuals.
Relative Sensitivity: 97.25%** (95%CI*: 92.17%-99.43%)*Confidence Intervals
Relative Specificity: 100%** (95%CI*: 97.16%-100%)
Accuracy: 98.73%**(95%CI*: 96.35%-99.74%)
Specimen: Nasal swab.
**Please Note. This is a manufacturer study comparing results for symptomatic subjects who were tested with both a PCR laboratory based test and this test kit to measure the test agreement. This Lateral flow test has been evaluated by Public Health England and found to reliably detect those with viral loads associated with infectiousness whether the test subject is symptomatic or asymptomatic. See the table below for Public Health England’s evaluation, Healgen is listed as the ‘Orient Gene’ test (the device manufacturer).
Public Health England Assessment Data for Healgen

Table 1. Results of the Public Health England Phase 3b evaluations showing viral antigen detection/sensitivity of LFD tests using dry-swab samples from community sampling. Healgen is listed as the ‘Orient Gene’ test. Tests were performed by laboratory scientists. Ct – cycle threshold on RT-PCR.
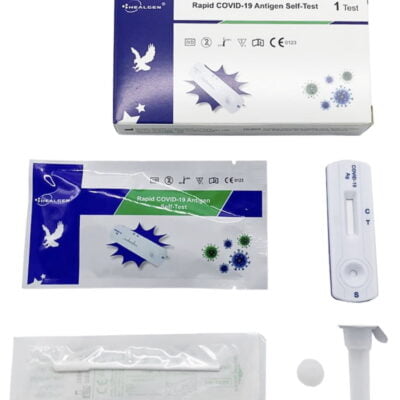
Pack Contents
- 20 Test Cassettes
- 20 Sterile Swabs
- 20 Pre-Filled (with buffer liquid) Extraction tubes and tips
- 2 WorkStations
- 1 Package insert
Nasal Swab Specimen Collection – sample of product instructions
How to perform a Nasal Swab

-
- Using the sterile swab provided in the kit, carefully insert the swab into one nostril of the patient. The swab tip should be inserted up to 2-4cm until resistance is met.
- Roll the swab 5 times along the mucosa inside the nostril to ensure that both mucus and cells are collected.
- Using the same swab, repeat this process for the other nostril to ensure that an adequate sample is collected from both nasal cavities.
- Withdraw the swab from the nasal cavity. The specimen is now ready for preparation using the extraction buffer provided in the test kit.
Sample preparation procedure
- Insert the test extraction tube into the workstation provided in the kit. Make sure that the tube is standing upright and reaches the bottom of the workstation.
- Tear off the sealing film on the extraction tube gently to avoid spilling out the liquid.

- Insert the swab into the extraction tube which contains 0.3 mL of the extraction buffer.
- Roll the swab at least 6 times while pressing the head against the bottom and side of the extraction tube.
- Leave the swab in the extraction tube for 1 minute.
- Squeeze the tube several times from the outside to immerse the swab. Remove the swab.

Test procedure
Allow the test device, test sample and buffer to equilibrate to room temperature (15-30 C) prior to testing.
- Just prior to testing remove the test device from the sealed pouch and lay it on a flat surface.
- Push the nozzle which contains the filter onto the extraction tube. Ensure the nozzle has a tight fit.
- Hold the extraction tube vertically and add 4 drops (approximately 100 µL) of test sample into the sample well.
- Start the timer
- Read the results at 15 minutes. Do not interpret the result after 20 minutes.
Interpretation of results

- POSITIVE: The presence of two lines as control line (C) and test line (T) within the result window indicates a positive result.
- NEGATIVE: The presence of only control line (C) within the result window indicates a negative result.
- INVALID: If the control line (C) is not visible within the result window after performing the test, the result is considered invalid. Some causes of invalid results are because of not following the directions correctly or the test may have deteriorated beyond the expiration date. It is recommended that the specimen be re-tested using a new test.
Quality control
A procedural control is included in the test. A red line appearing in the control line region (C) is the internal procedural control. It confirms sufficient specimen volume and correct procedural technique.
Frequently Asked Questions
-
- What does antigen mean?
An antigen is a foreign substance that induces the body to produce antibodies. In this context, the antigen belongs to SARS-CoV-2, the virus that causes COVID-19. Specifically in this case, the target protein ‘antigen’ is the N-Protein of this member of the Coronavirus Family. This N-Protein surrounds the genetic material within the sphere of the viral particle.
- What does Lateral Flow mean?
A Lateral Flow device is a type of diagnostic test which uses a pre-prepared liquid sample that is placed onto a cassette with an absorbent pad in it. Once the liquid sample is absorbed the sample reacts with the antibodies and reactive molecules and displays the result as a set of coloured lines. The liquid sample flows laterally along the test strip, hence the name Lateral Flow Test.
- What is antigen testing?
Antigen tests detect proteins specific to a virus that appears in infected individuals, which indicates active infection. Rapid antigen testing is beneficial because of their portability, ease-of-use, and swift time to result.
- What is specificity?
- What does antigen mean?
Specificity refers to how specific the positive result of the test is to being activated by the Covid-19 Virus Target Antigen. If a test has a high specificity there will be a low number of false positive results. False positives in any test can be caused by other pathogens reacting with the test to cause a positive result to show. Our tests are checked for cross-reactivity against other seasonal coronaviruses and high specificity is one of the conditions of the UK Government Approval.
- What is sensitivity?
Sensitivity is the measure of the ability of the test to pick out a true positive result. This is usually relative to a PCR Laboratory-based test. The Healgen COVID-19 Test has very high sensitivity at Viral Loads associated with infectiousness.
- Whats the difference between this antigen test and a PCR Test?
A PCR Test is a molecular based test, as opposed to the antigen test method above PCR works by analysing the genetic code contained at the centre of the virus in a laboratory. The Polymerase-Chain-Reaction test runs in thermal cycles, amplifying the genetic material each cycle until large enough for the result to be detected visibly by the laboratory operator by means of a colour change. PCR Tests can detect people who have a lower level of virus in the body before they are significantly infectious but are more expensive than antigen tests due to requiring laboratory processing. One disadvantage of the PCR test method is it can often detect viral fragments several weeks after a person is no longer able to infect people.
- What is the extraction buffer?
The extraction buffer includes components to dissociate viral proteins from their surfaces to be used as a target for the test. The method of the extraction buffer is used to prepare the test sample by dissolving the virus shell, which also deactivates the virus. This presents the N-Protein to the test strip.
Shipment Tracking Available
 *When ordering 2 or more 20 packs of Healgen Tests.
*When ordering 2 or more 20 packs of Healgen Tests.






Reviews
There are no reviews yet.